Revolutionary Therapy Clears 13-year-Old Girl’s Incurable Cancer
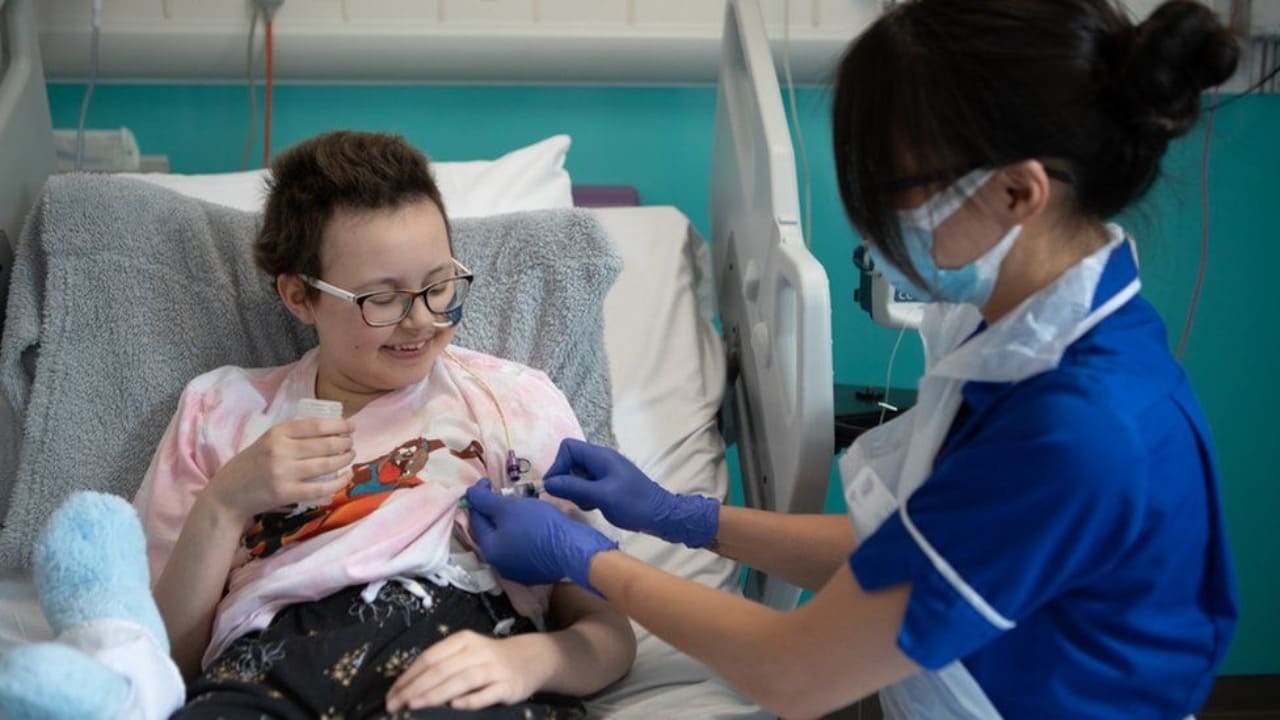
After a teenager became the first patient to receive the revolutionary medication and later enter remission, doctors in Britain lauded the groundbreaking treatment for an aggressive form of leukemia.
The 13-year-old girl, known only as Alyssa, was identified as having T-cell acute lymphoblastic leukaemia in 2021.
But conventional therapy, such as chemotherapy and a bone marrow transplant, had little effect on her blood malignancy.
She was enrolled in a clinical study for a groundbreaking therapy at London's Great Ormond Street Hospital for Children (GOSH), which used genetically modified immune cells from a healthy volunteer.
Her cancer went into remission after 28 days, which gave her the opportunity to get a second bone marrow transplant to rebuild her immune system.
She is "doing well" at home in Leicester, central England, six months after the incident and is getting follow-up care.
The hospital stated in a statement on Sunday that palliative care was Alyssa's only choice absent this experimental therapy.
Her recovery was "very extraordinary," according to Robert Chiesa, a GOSH consultant, albeit the outcomes still needed to be watched and verified over the coming few months.
The most prevalent kind of cancer in kids is acute lymphoblastic leukemia (ALL), which affects the B and T cells of the immune system, which defend and combat pathogens.
Alyssa was the first patient to receive base-edited T cells, according to GOSH, which entails chemically modifying single nucleotide bases, which are letters in the DNA code and carry instructions for a particular protein.
In 2015, GOSH and University College London researchers contributed to the development of the use of genome-edited T cells to treat B-cell leukemia.
However, the scientists faced a problem while trying to treat some other forms of leukemia because T cells made to identify and combat malignant cells turned out to be killing one another in the manufacturing process.
The base-edited cells required multiple more DNA alterations to enable them to target malignant cells without harming healthy cells.
The GOSH consultant immunologist and professor Waseem Qasim stated, "This is a remarkable indication of how, with competent teams and infrastructure, we can integrate cutting-edge technology in the lab with genuine results in the hospital for patients."
It is the most advanced cell engineering we have done to date and opens the door to more innovative therapies and ultimately brighter futures for sick youngsters.
According to Alyssa's statement, she was motivated to participate in the trial not just for herself but also for other kids.
"Hopefully this can prove the study works and they can offer it to more youngsters," her mother Kiona added.




